Water treatment for pharma & biotech
A complete and unique range of technologies and services for the treatment of purified water and wastewater. Discover the effect of obtaining ultrapure water for the pharmaceutical and biotechnology industries.
Purifying water
By referring to the European Pharmacopoeia (Ph. Eur.) guideline on the quality of water for pharmaceutical use.
Water for injections (WFI)
Water for the preparation of medicines for parenteral administration when water is used as a vehicle (water for injections in bulk) and for dissolving or diluting substances or preparations for parenteral administration (sterilised water for injections).
PurifiedWater (PW)
water for the preparation of medicines other than those that are required to be both sterile and apyrogenic, unless otherwise justified and authorised.
Water for preparation of extracts
water intended for the preparation of Herbal drug extracts .
Applications in the pharmaceutical and biotechnology sectors.
Ensure a constant and reliable supply of purified water at the required quality level
Installing closed-loop and/or turnkey systems equipped with a range of purification technologies
Provide simple to use, easy to maintain systems based on decades of experience
01.
Pretreatment
Regardless of the level of quality required, the raw water source is usually that of the drinking water network or a local resource such as borehole water. This water, although it must meet strict drinkability criteria, is not suitable as such for pharmaceutical applications. Water intended for human consumption contains elements that are undesirable, either for the production process or to achieve the necessary level of quality.
WTT, thanks to its knowledge of the field and its know-how, does what is necessary to carry out a pre-treatment allowing to have water that meets the needs of the chosen producer, and olso in order to keep reverse osmosis membranes efficiency and long lasting. According to the origin, quality and use of feed water, solutions might consist in providing suitable filters, softeners, dosing systems, heat ex changers…
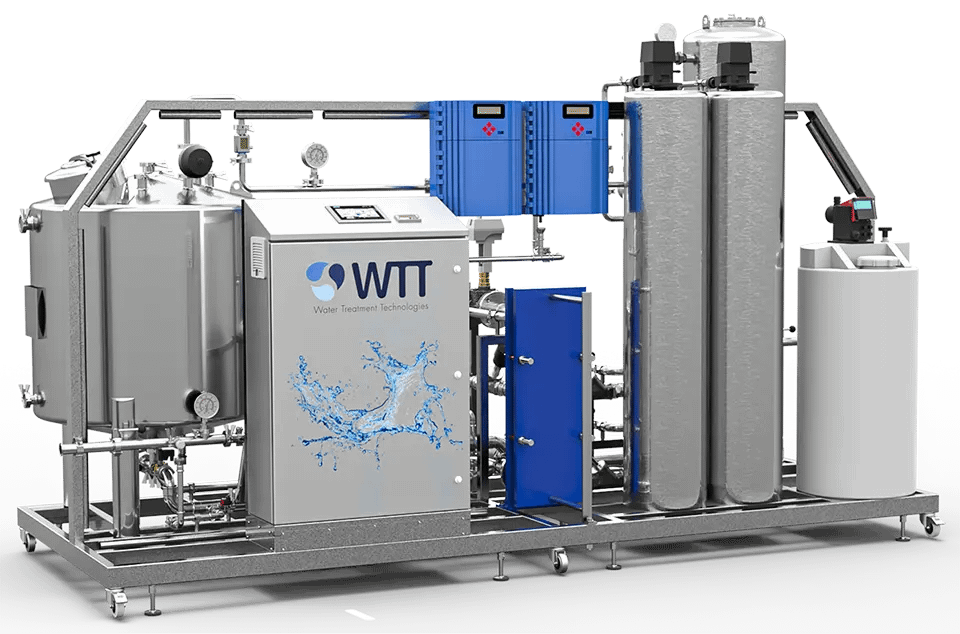
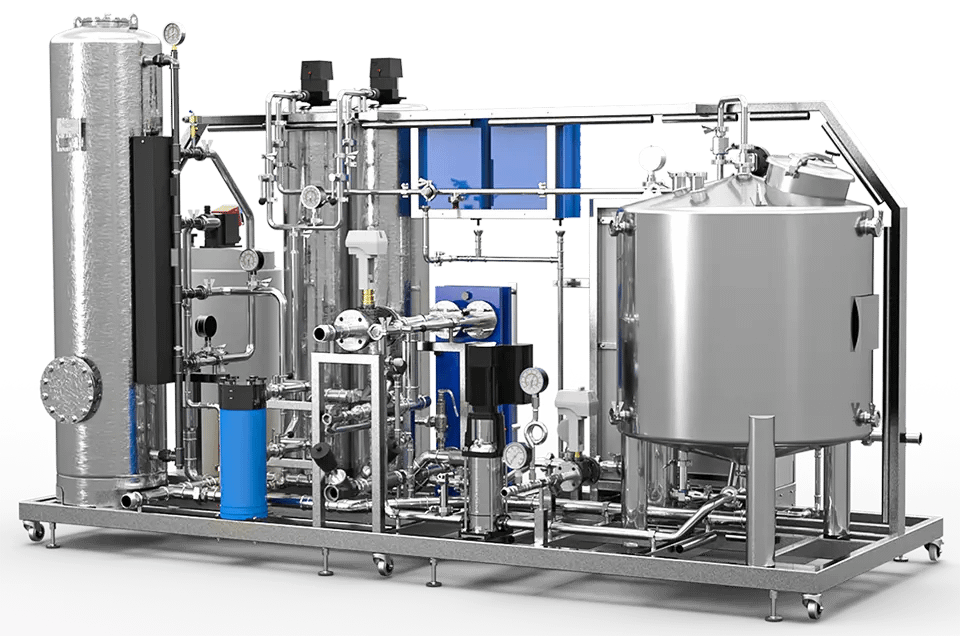

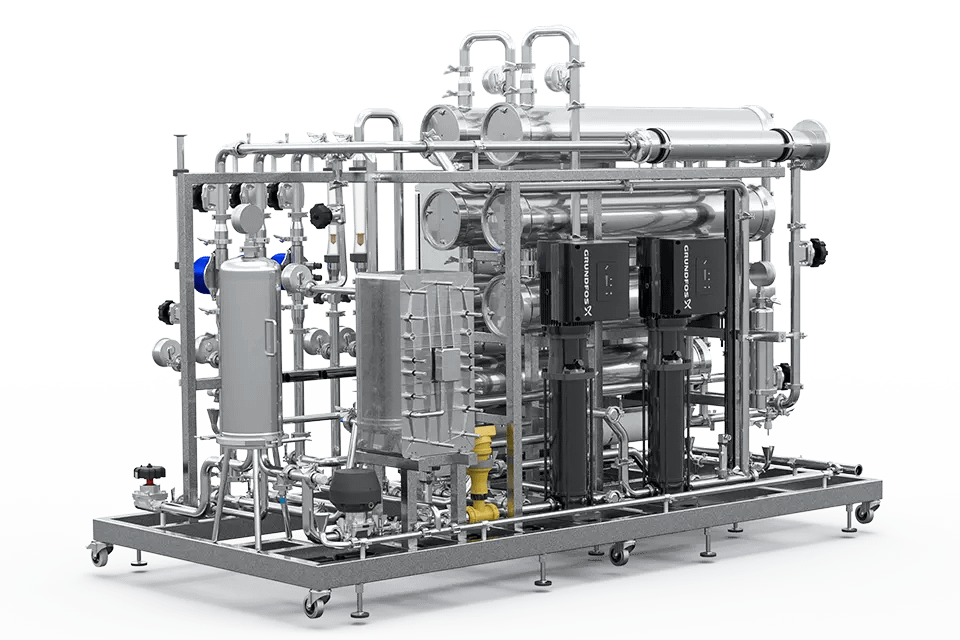
02.
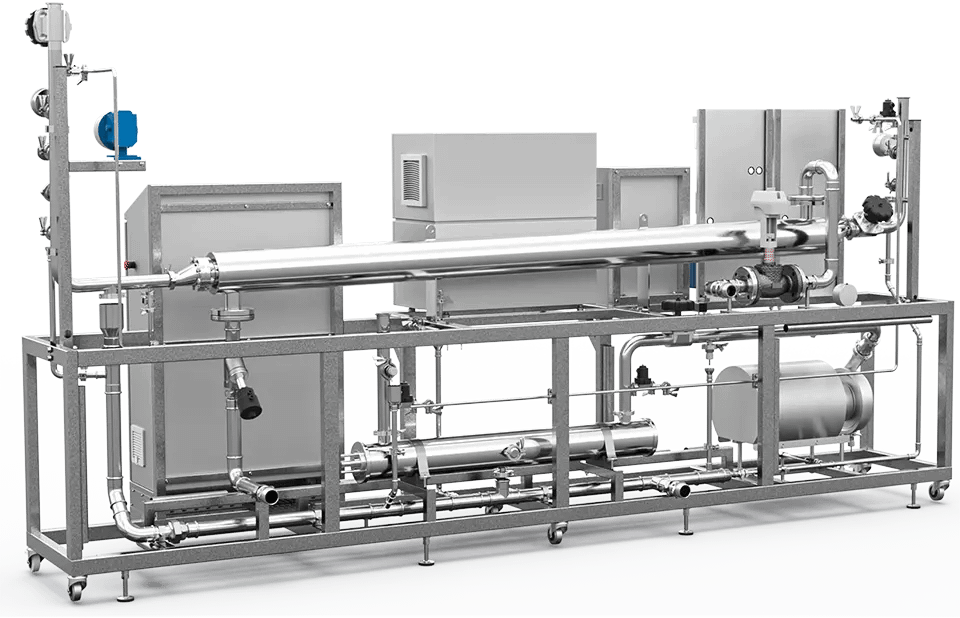
Hydropharma
Producing PW and cold WFI for pharmaceutical and biotechnology product manufacturers according to the European and American pharmacopoeia.
Confirm to the guidelines, HYDROPHARMA is based on the membrane systems such as a single or a double reverse osmosis (RO) combined with continuous electrodeionization (CEDI) for the production of PW, and ultrafiltration (UF) membrane-system to remove any biofilm growth or endotoxins for the production of cold WFI.
03.
Hydroloop
WTT is putting in place the means to make water for pharmaceutical use available at points of use (POU), while maintaining its quality.
Includes tanks, pumps, heat exchangers , and UV, ozone generators (by electrolysis) and also installs the management and monitoring elements (conductivity, TOC, Temperature.. ).
WTT takes care that all the elements in contact with water for pharmaceutical use are of high quality and homogeneous roughness: tanks, pipes, etc. The choice of surface quality is linked to the technologies implemented in terms of disinfection and sterilization.
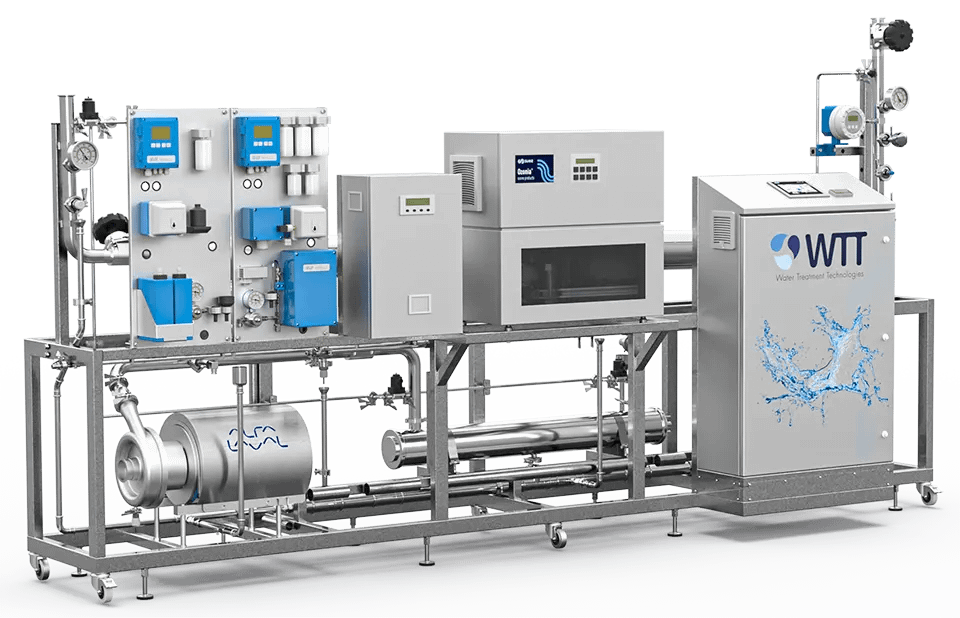


06.
Sanitization
Water Treatment Technologies use one of the most effective and safeway to disinfect Ultra-Pure Water loops in industrial and pharmaceutical applications: the electrolytic ozone generators. The residual ozone is destroyed by a sanitary UV system.
The quantity of ozone is controlled at the purified water tank outlet and the UV system. WTT can also provide a chemical or a heat sanitization which requires membranes and EDI modules suitable for hot water circulation.
07.
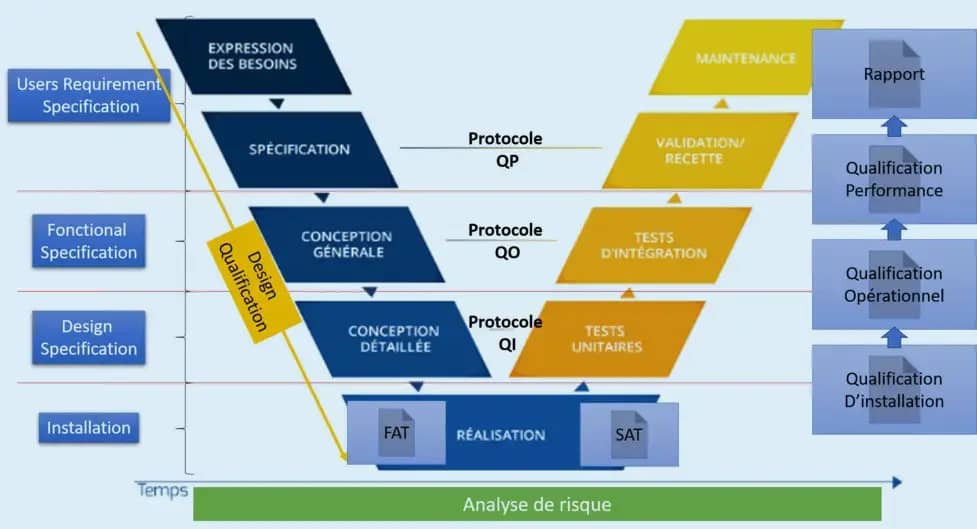
Validation
As a general rule, the matrix of the qualification master plan is established and finalized in parallel with the phases of the call for tenders, the offer and the order (by the customers) .WTT carries out the Conceptual
Qualification (DQ) which defines and gives documented proof that the required quality is taken into account. The Conceptual Phase (DQ) forms the basis for future activities that will be carried out during Installation Qualification (IQ) and Operational (OQ).
WTT performs Installation Qualification (IQ) which defines and provides documented demonstration that equipment and systems are installed in accordance with approved plans, technical specifications and legal safety guidelines. Carried out by WTT after the commissioning of the entire installation, the Operational Qualification (OQ) defines and provides documented proof that the equipment and systems are functioning as intended. After the Operational Qualification (OQ), the Performance Qualification (QP) which is the customer’s responsibility, checks the capacity of the equipment and the system.
“We appreciate the level of expertise and support your team provides, and we highly recommend your services to anyone in the industry”

Sanofi – Executive Vice President, Manufacturing and Supply
“They took the time to understand our processes and recommended a system that has exceeded our expectations.”

Sanofi – Executive Vice President, Manufacturing and Supply
We roam in the very essence of life and we see that everything is made out of water.
WTT is beyond water since 1996
The most professional and ethical, leading quality and cost effective water treatment technologies services and products provider.
Working with trusted partners







